Secukinumab Gains FDA Approval to Treat Hidradenitis Suppurativa
11/15/2023
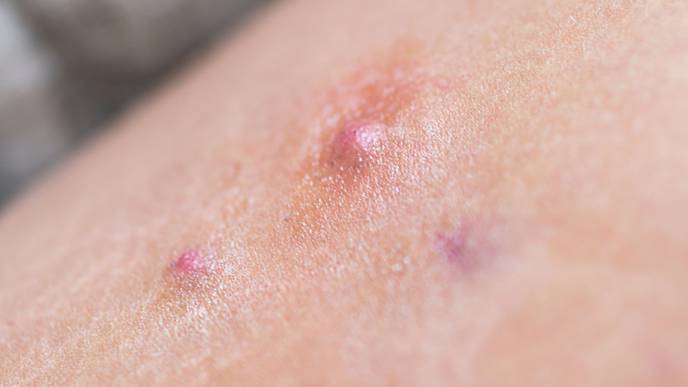
FDA has approved secukinumab (Cosentyx) to treat moderate to severe hidradenitis suppurativa (HS) in adults. The treatment is the second biologic approved to treat HS, a chronic, systemic, and often painful skin disease. Symptoms include recurring boil-like lumps that may burst into open wounds and cause irreversible scarring.
The approval was based on the results of the phase 3 trials SUNSHINE and SUNRISE.
“For many patients, the daily impact of HS and the search for symptom relief can last years – which can come with painful, irreversible physical and emotional scarring,” Alexa B. Kimball, MD, MPH, lead investigator of the SUNSHINE and SUNRISE trials, professor of dermatology at Harvard Medical School, president and CEO of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center, Boston, said in a statement. “This approval marks an important milestone for countless patients who have been faced with limited treatment possibilities and who now have a new option.”

Facebook Comments