Benefit/Risk Strategies in Selecting Therapeutic Solutions for MS

Benefit/Risk Strategies in Selecting Therapeutic Solutions for MS
Benefit/Risk Strategies in Selecting Therapeutic Solutions for MS: HCP and Patient Viewpoints
Learning Objectives
- Review the benefit/risk strategies in selecting therapy for MS patients while assessing treatment regimens that carry acceptable or diminished risk of disease progression
- Explore emergent concepts in the management of MS, focusing on targeting T- and B-cells including:
- Risks associated with continuous immunosuppression
- Action on the inflammatory activity in the CNS compartment
- Identify strategies that simplify patient dosing and side effects to:
- Increase treatment compliance
- Improve patients’ quality of life
- Slow disease progression
As Disability of MS Advances, Work Capacity Decreases

The proportion of MSers employed or on long-term sick leave is calculated as a percentage of MSers aged 65 or younger.
1. Kobelt G et al. J Neurol Neurosurg Psychiatry. 2006;77:918-926; 2. Pfleger CC et al. Mult Scler. 2010;16:121-126.
The Traditional Approach to MS Treatment

- Heterogeneity of disease course across different MSers and over time can affect treatment response1-3
- Depending on the definition used, up to 49% of MSers treated with a first-line injectable therapy (IFNB) still have clinical disease activity1
1. Rio J et al. Ann Neurol 2006;59:344-52; 2. Miller A et al. J Neurol Sci 2008;274:68-75; 3. Rudick RA et al. Lancet Neurol 2009;8:545-59.
Figure adapted from Rio J et al. Curr Opin Neurol 2011; 24:230-7.
Treating Beyond Symptoms with a View to Improving Outcomes in Inflammatory Bowel Diseases

T2T-NEDA ALGORITHM

- MS prognosis based on clinical and MRI indices
- Life style and goals
- Shared goals for therapy
- Patient’s preferences?
- Your choice?
- Only one licensed induction therapy at present
Rebaselining:
- ifn-β, natalizumab, fingolimod, teriflunomide, dimethyl-fumarate=3-6 months
- glatiramer acetate=9 months
- alemtuzumab=24 months
Individual measures:
- Evidence of disease activity?
- Tolerability/safety?
- Adherence?
- Drug or inhibitory markers, e.g. NABs?
Interferon-beta Reduced Mortality by 46.8% vs Placebo Over 20 Years

Inflammation Drives Acute Axonal Loss and Primes Surviving Axons for Degeneration Later

Treatment Effect on Disability Predicted by Effect on T2-lesion Load and Brain Atrophy

Meta-analysis of treatment effect on EDSS worsening (y) vs effects on MRI lesions and brain atrophy, individually or combined, in 13 placebo-controlled RRMS trials (13,500 patients)
No Evident Disease Activity: NEDA

Gd, gadolinium.
1. Havrdova E, et al. Lancet Neurol. 2009; 8:254–260; 2. Giovannoni G, et al. Lancet Neurol 2011; 10:329–337.
MS Pyramid

Risk vs Benefit

Theoretical Model: Treat Early and Effectively
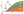
Early – Highly Active Treatment Enhances Outcome

Cost of Delayed Access to Highly Active Treatment
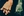

Large Disparities Exist Among Countries in Access to Disease-Modifying Therapies


All data are from 2013
Established DMTs
DMTs approved for relapsing forms of MS during the 1990s and reformulations or generic versions of these substances
Newer DMTs
DMTs approved for relapsing forms of MS that have a different mechanism of action from established DMTs
DMT, disease-modifying therapy.
1.Hollingworth S et al. J ClinNeurosci2014;21:2083–7; 2. World Bank, 2015. http://data.worldbank.org/indicator/SP.POP.TOTL ; 3. MSIF, 2013. http://www.atlasofms.org ; 4.Wilsdon T et al. 2013. http://crai.com/sites/default/files/publications/CRA-Biogen-Access-to-MS-Treatment-Final-Report.pdf . Figure reproduced from Giovannoni G et al. Brain health: time matters in multiple sclerosis. Available at: www.msbrainhealth.org
Multiple Sclerosis: Unmet Medical Needs
- Disease-modifying drugs (DMDs) are not completely effective in all patients.
- 7 to 49% of relapsing–remitting MS (RRMS) patients do not adequately respond to DMDs
- Current Options Injection/Infusion
- Needle phobia (25% of population)
- Clinic infusion visit required
Rudick RA, Polman CH. Lancet Neurol. 8, 545–559 (2009).
The Goal of Treating MS Should Be to Maximize Lifelong Brain Health

Our Vision Is to Create a Better Future for People with MS and Their Families
Your voice will help to effect this change.
Be an early adopter
Pledge your support of the report’s recommendations at www.msbrainhealth.org
Therapeutic Hierarchy

Strategies to Reduce Time Spent with the Clinician and Enhance Adherence
- Dosing Schedule – 10 days annually for 2 years
- Oral administration – More appealing than needles
- Low Discontinuation Rate – Less anxiety for the patient and demand for HCP time
- Less Monitoring – Depends on the progression of MS and patient specific needs
Evolution In Disease Modifying Drugs For Relapsing Remitting Multiple Sclerosis

Disease modifying drugs: Benefit/risk evaluation
Established Inconveniences and Risks
- Convenience
- Monitoring
- Tolerability
- Safety
Established Benefits
- Treatment efficacy

Interferon Beta: Benefit/Risk Evaluation
Established Inconveniences and Possible Risks
- Injectable
- Frequent s.c. or i.m. injections
- Trivial side effects
- Flu-like symptoms (IFNβ)
- Injection site reactions
- Neutralizing Antibodies (Nabs)
Established Benefits
- Moderate effect on disease activity (on average 30% reduction in relapse rate)
- Less effect on disability progression
- Excellent response in approximately 30% of patients
- No long-term safety concerns
Glatiramer Acetate: Benefit/Risk Evaluation
Established Inconveniences and Possible Risks
- Injectable
- Daily injections may decrease adherence
- Trivial side effects
- Injection site reactions
- Systemic reactions
Established Benefits
- On average a moderate effect on disease activity (30% reduction in relapse rate)
- Less effect on disability progression
- Excellent response in approximately 30% of patients
- No long-term safety concerns
Teriflunomide: Benefit/risk Evaluation
Established Inconveniences and Possible Risks
- Adverse effects
- Diarrhea and nausea
- Hair thinning
- ALT increase
- Potentially immunosuppressive properties
Established Benefits
- Moderate effect on disease activity
- Moderate effect on disability progression
- Equal to IFN-β 1a SC
- One tablet daily
Dimethyl Fumarate: Benefit/Risk Evaluation
Established Inconveniences and Possible Risks
- Adverse effects
- Flushing
- Abdominal pain
- Administered as two tablets daily
- Low risk of PML
Established Benefits
- Robust effect on disease activity
- Moderate effect on disability progression
- Numerically but not statistically significant better than GA
Fingolimod: Risks/Inconveniences > Benefits
Established Inconveniences and Possible Risks
- Adverse effects
- Bradycardia, A-V block
- Retinal edema
- Infections: dermatomal zoster
- Infrequent severe adverse effects
- Serious infections: disseminated varicella†, herpes encephalitis†
- Skin cancers
- Single case of PML
Established Benefits
- Superior to IFN-β 1a
- Large effect on disease activity
- Moderate effect on disability progression
- One table daily
Natalizumab: Benefits > risks/inconveniences
Established Inconveniences and Possible Risks
- Intravenous infusions
- Rare infusion reactions
- Rare Nabs
- Infrequent severe adverse effects
- PML in 2:1000 per year (after 2 years)
Established Benefits
- Profound effect on disease activity
- Significant effect on disability progression
- Improves QoL
- Good cost-effectiveness
- Risk stratication for PML possible
Alemtuzumab: Benefits > risks/inconveniences
Established Inconveniences and Possible Risks
- Infusion associated reactions
- Infections
- Immune thrombocytopenic purpura
- Immune thyroid disorders
- Immune nephropaties
- Cytopenias
Established Benefits
- Robust effect on disease activity and disability progression
- Infrequent administration
- Long-lasting efficacy
- Superiority to IFN-β 1a sc
75-81% of Patients Treated in CLARITY were Relapse-Free after 2 Years vs No Additional Treatment

CLARITY EXT: Patients Free from Evidence of MRI Activity

Proportion with no new T1 Gd+ lesions in the cladribine 3.5 mg/kg group
Giovannoni G et al. ECTRIMS Abstract 553 September 2016.
CLARITY: Effects of Cladribine 3.5 mg/kg on Time to 6-Month Confirmed EDSS Progression

CLARITY: Benefits of Cladribine on MRI Outcomes in Pooled Double-Blind Data - T1 gd+ lesions

Effects of cladribine 3.5 mg/kg vs placebo on the relative risk ratio of cumulative new T1 gd+ lesions in patient subgroups.
CLARITY: Benefits of Cladribine on MRI Outcomes in Pooled Double-Blind Data – T2 lesions

Effects of cladribine tablets 3.5 mg/kg vs placebo on the relative risk ratio of cumulative active T2 lesions in patient subgroups
CLARITY: Brain Volume Loss in Patients with Relapsing MS

- 3.5 mg/kg or 5.25 mg/kg showed significantly less brain atrophy than placebo.
- Brain volume changes showed a correlation between brain atrophy and disability progression
- Treatment with cladribine tablets was associated with a significantly lower risk of disability progression compared with placebo.
Stefano ND et al. ECTRIMS Abstract 547 September 2016.
ORACLE-MS: Long-Term Follow-Up Analysis of Patients
Worst Post-Baseline CTCAE Grade in Patients Not Treated During Long Term Follow Up
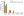
Leist T et al ECTRIMS Abstract 609 September 2016.
Conclusions
- MS is a disease that has far-reaching negative implications
- Mortality, disability, unemployment, divorce, suicide cognitive impairment, etc.
- Era of Individualised Profiling
- Prognosis, risk, treatment and monitoring
- New treatment paradigm
- Maintenance vs. induction therapy
- Early highly-effective treatments are now a first-line option
- Improved risk mitigation tools
- New treatment paradigm of treat-2-target of NEDA (No Evidence of Disease Activity)
- Is it fair to make patients wait 20 years for the outcome of an ongoing experiment?
This resource is supported by an educational grant from Merck KGaA, Darmstadt, Germany.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended


Treatment of Progressive MS: Why, Who, When, How
Treatment of Progressive MS: Why, Who, When, How
ResourcesTreatment of Progressive MS: Why, Who, When, How


Now Approved in Europe: First Oral Short-Course Treatment for Highly Active Relapsing MS
Now Approved in Europe: First Oral Short-Course Treatment for Highly Active Relapsing MS
ResourcesNow Approved in Europe: First Oral Short-Course Treatment for Highly Active Relapsing MS


Cladribine Receives Positive CHMP Opinion for Relapsing MS Treatment
Cladribine Receives Positive CHMP Opinion for Relapsing MS Treatment
ResourcesCladribine Receives Positive CHMP Opinion for Relapsing MS Treatment


Reducing the Burden of MS Treatment
Reducing the Burden of MS Treatment
ResourcesReducing the Burden of MS Treatment


